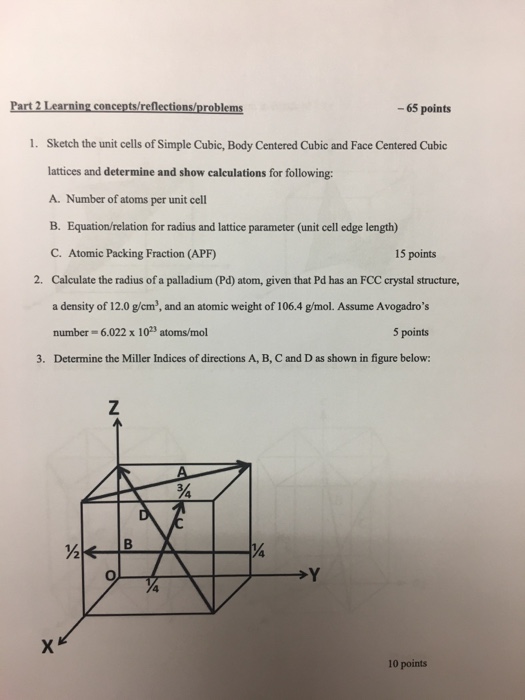
An element has a body-centered cubic (bcc) structure with a cell edge of 288pm. The density...... - YouTube

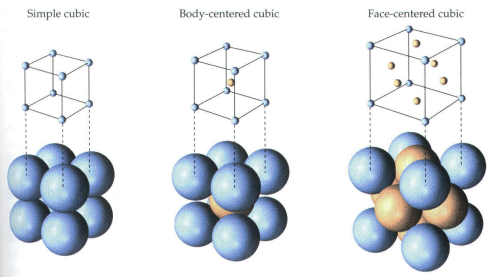
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu - YouTube

Unit Cell Chemistry, Atomic Radius, Density & Edge Length Calculations, Close Packed Structures - YouTube

Niobium crystallizes in body-centered cubic structure. If density is 8.55 g/cm3, Calculate...... - YouTube

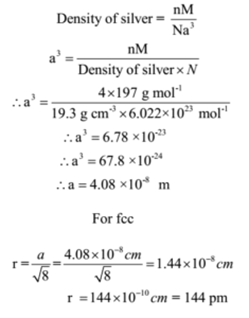
Silver crystallises in a face - centred cubic in cell. The density of Ag is 10.5 g cm^-3 . Calculate the edge length of the unit cell.

Unit Cell Chemistry, Atomic Radius, Density & Edge Length Calculations, Close Packed Structures - YouTube

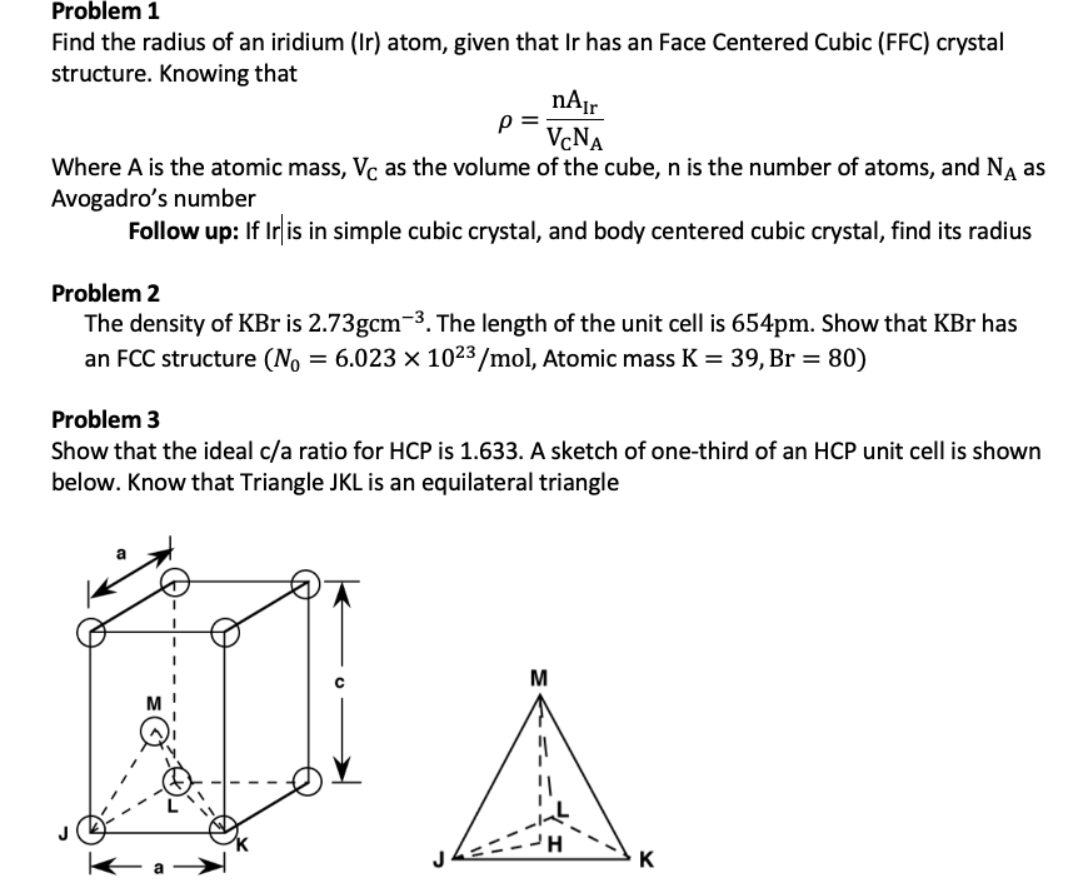
Niobium has a density of 8.57 g/cm3 and crystallizes with the body-centered cubic unit cell. Calculate the radius of a niobium atom - Chemistry Stack Exchange

SOLVED: Lead (atomic radius = 175 pm) crystallizes in a face-centered cubic unit cell. Calculate the density of lead given that in face-centered cubic, the edge length of a unit cell =

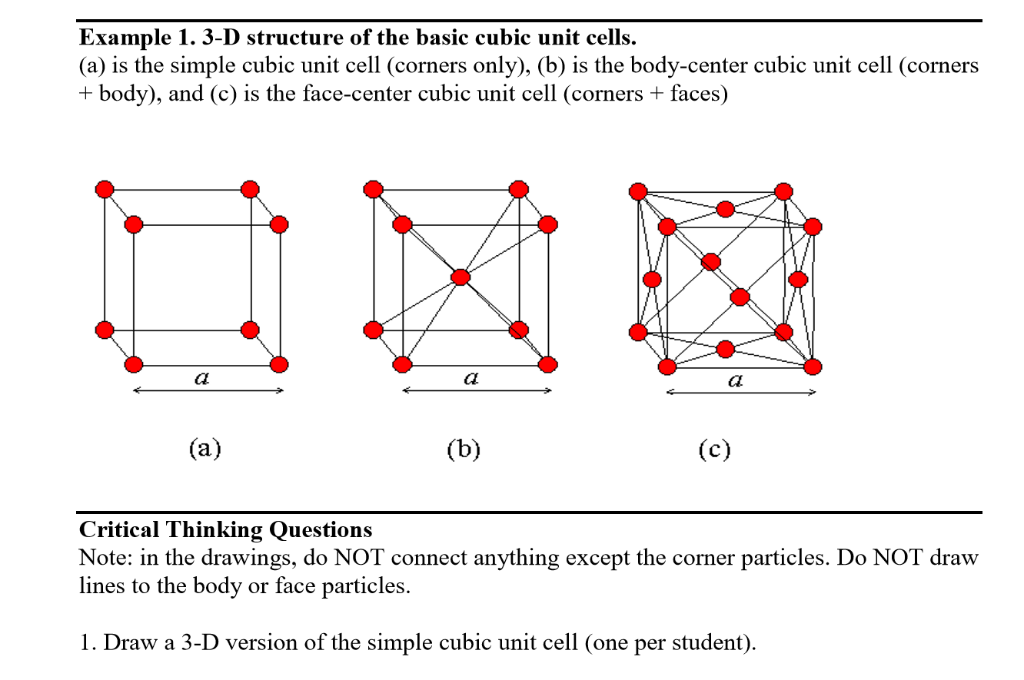
SOLVED: Determine the volume density of the atom in crystals with (a) simple -cubic,(b) body-centered cubic and(c) face-centered cubic crystal structures with a lattice constant a=5A.

Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu - YouTube
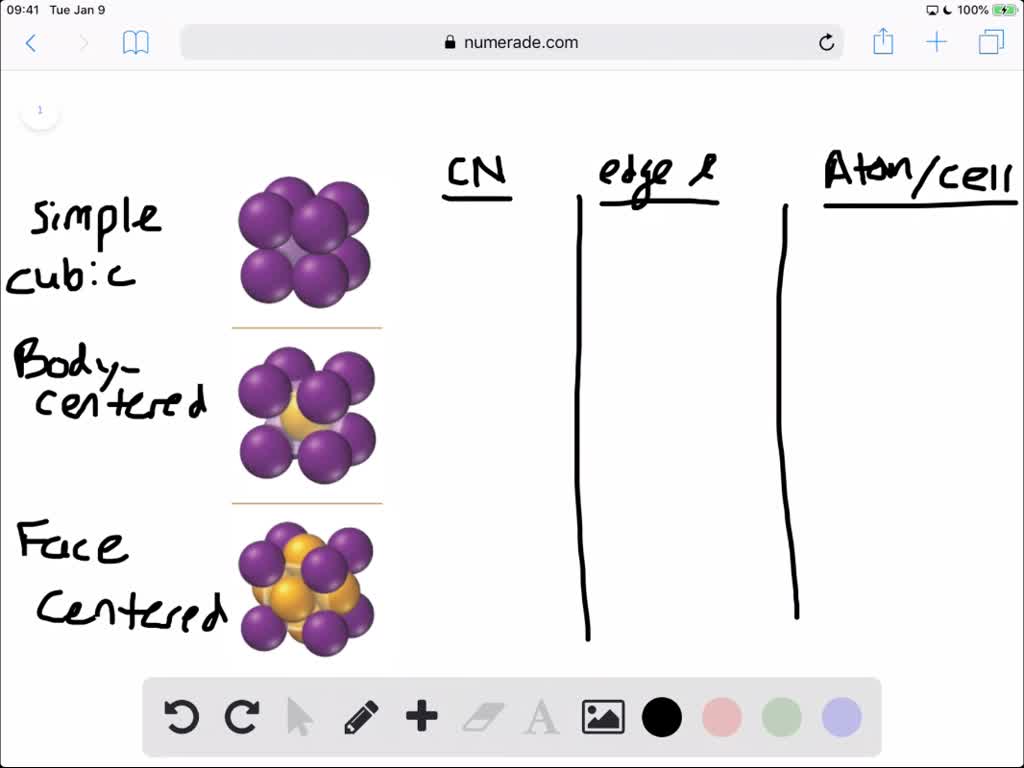
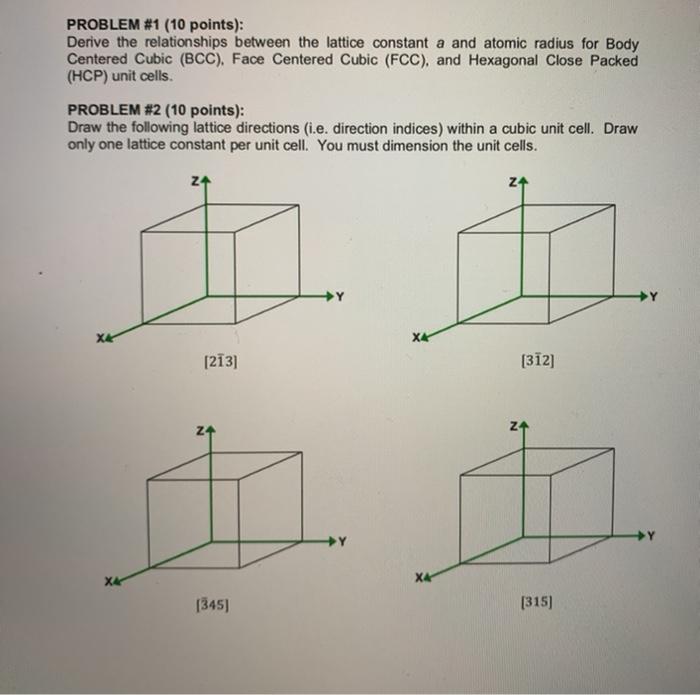
SOLVED:For each of the cubic cells in the previous problem, give the coordination number, edge length in terms of r, and number of atoms per unit cell.
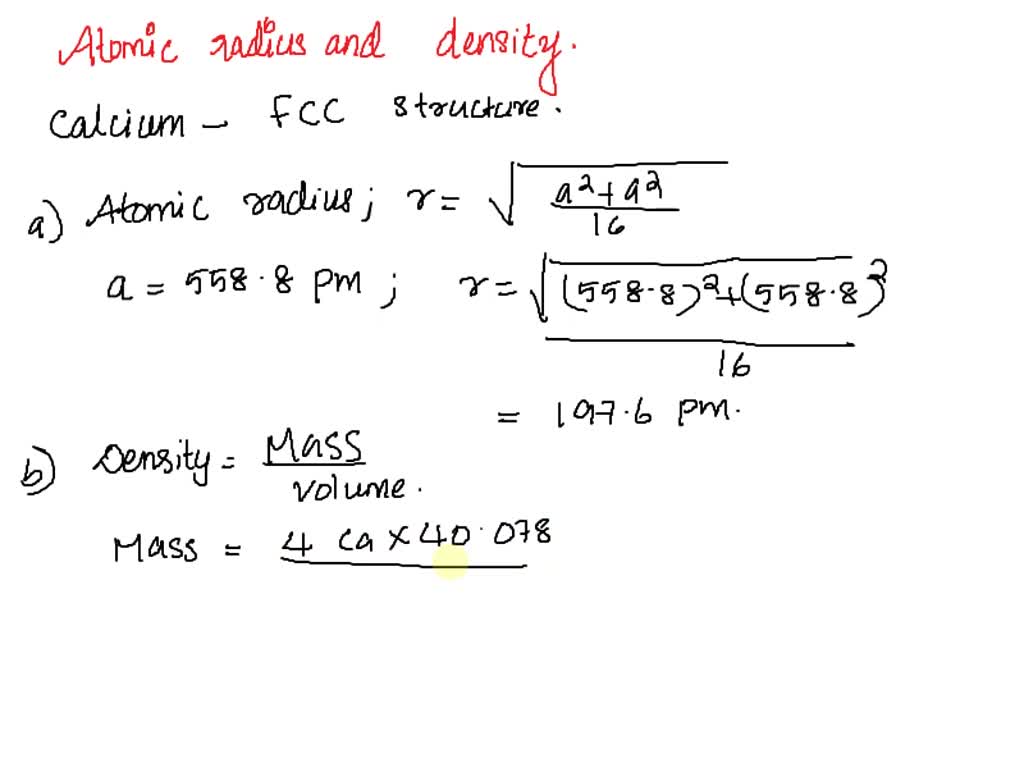

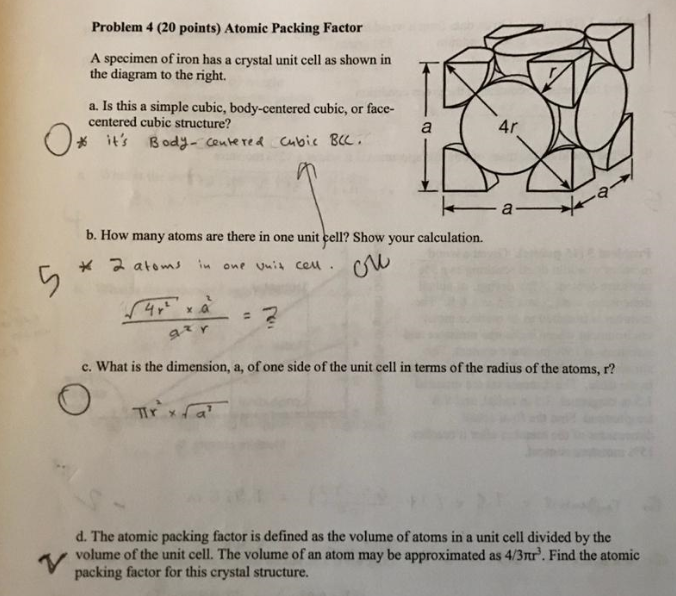



![Solved 3. Face Centered Cubic Structure [10 pts] Platinum is | Chegg.com Solved 3. Face Centered Cubic Structure [10 pts] Platinum is | Chegg.com](https://media.cheggcdn.com/media/1eb/1eb9fa27-1dc7-4453-878c-a7f39a79cbf4/phppeImhN.png)